
Restricted Access Barrier System (RABS) is a rigid barrier consisting of transparent walls (polycarbonate or glass) and outfitted with a suitable number of glove flanges and gloves. They utilize a mix of physical and aerodynamic barriers to prevent c...

VHP decontamination systems are becoming increasingly important as regulators turn their focus away from the frequently used Steam and Dry Heat Sterilization methods in order to reduce the risk of cross-contamination and bio-burden. The VHP Passbox...

A Containment Strategy is a method for controlling workplace hazards that has been approved by the HSE and the occupational hygiene profession. It covers both the managerial concerns and the engineering solutions required for the secure handlin...

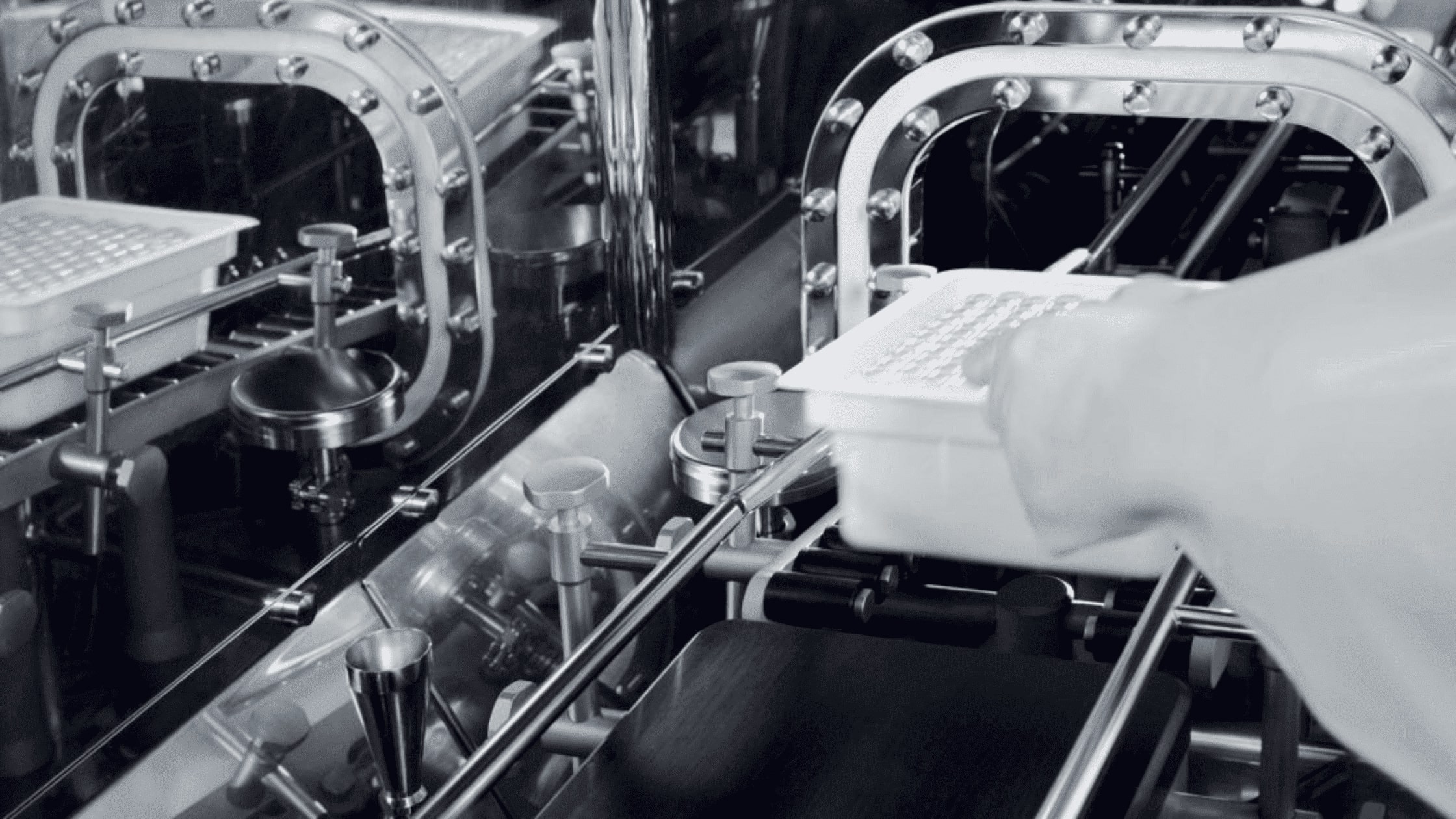
In the realm of pharmaceuticals, precision and safety are paramount. One critical process that underscores this principle is compounding aseptic containment. To achieve impeccable sterility and minimize the risk of contamination, the compounding asep...

APIs are the compounds utilized in the production of pharmacological medications. The active ingredient (AI) are the chemicals or substances that are biologically active inside the medicine and are the precise components responsible for the intended ...

Benefits of Automation in Pharmaceutical Containment Systems Containment solutions like isolators, glove boxes, and biosafety cabinets are mission-critical equipment in pharmaceutical manufacturing, biotechnology, and life-science research. In the pa...
Relative humidity and absolute humidity are two different concepts, although they both refer to the amount of water vapor in the air. Let us understand what exactly is Humidity and Relative Humidity. Absolute humidity: The whole mass of water vapor i...
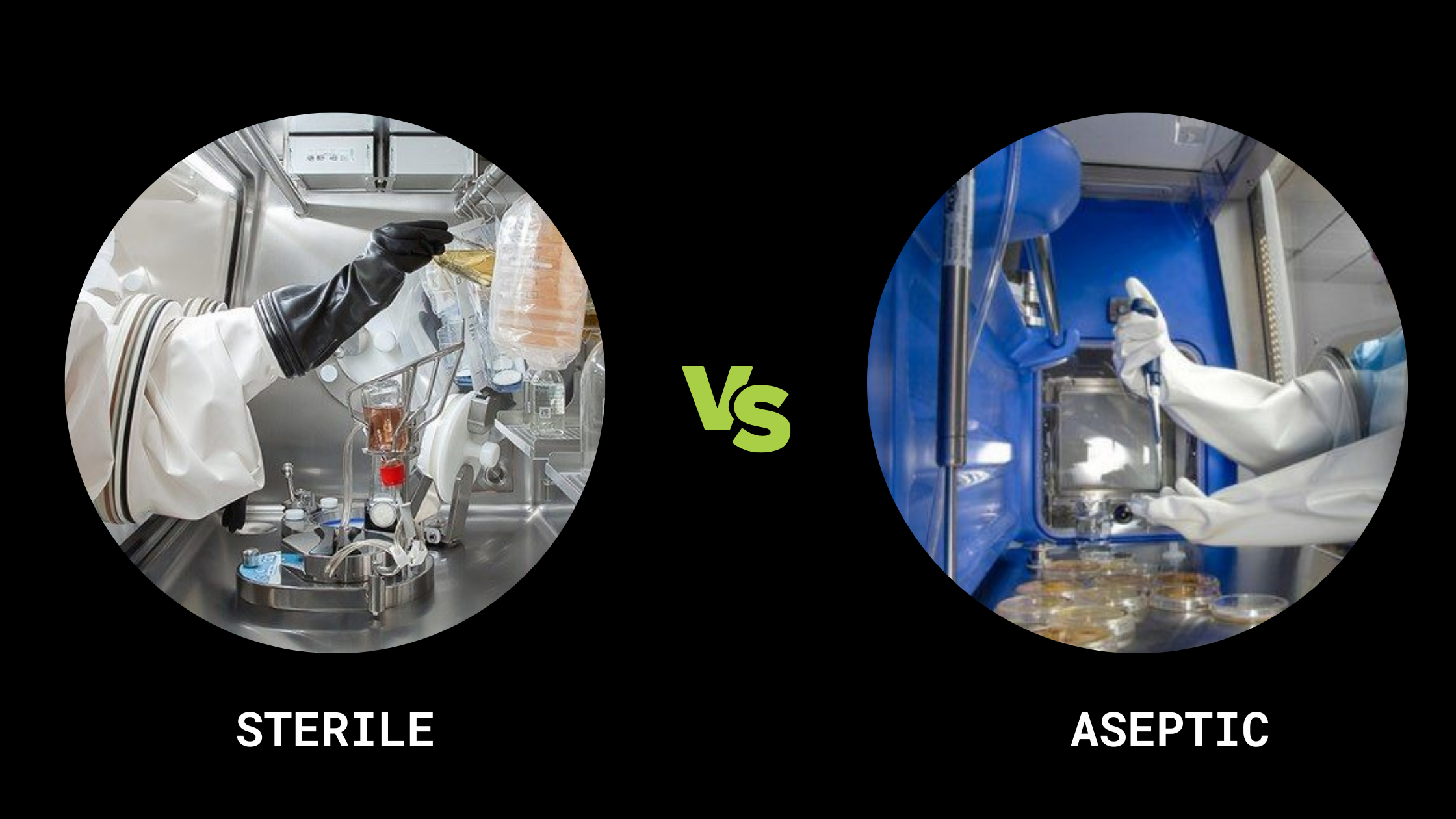
It ought to be obvious saying that in order to reduce the danger of infection, microbial cultures must be handled carefully. Therefore, it is crucial to comprehend the meaning of every terminology used in the explanation of safety measures. ‘Steril...
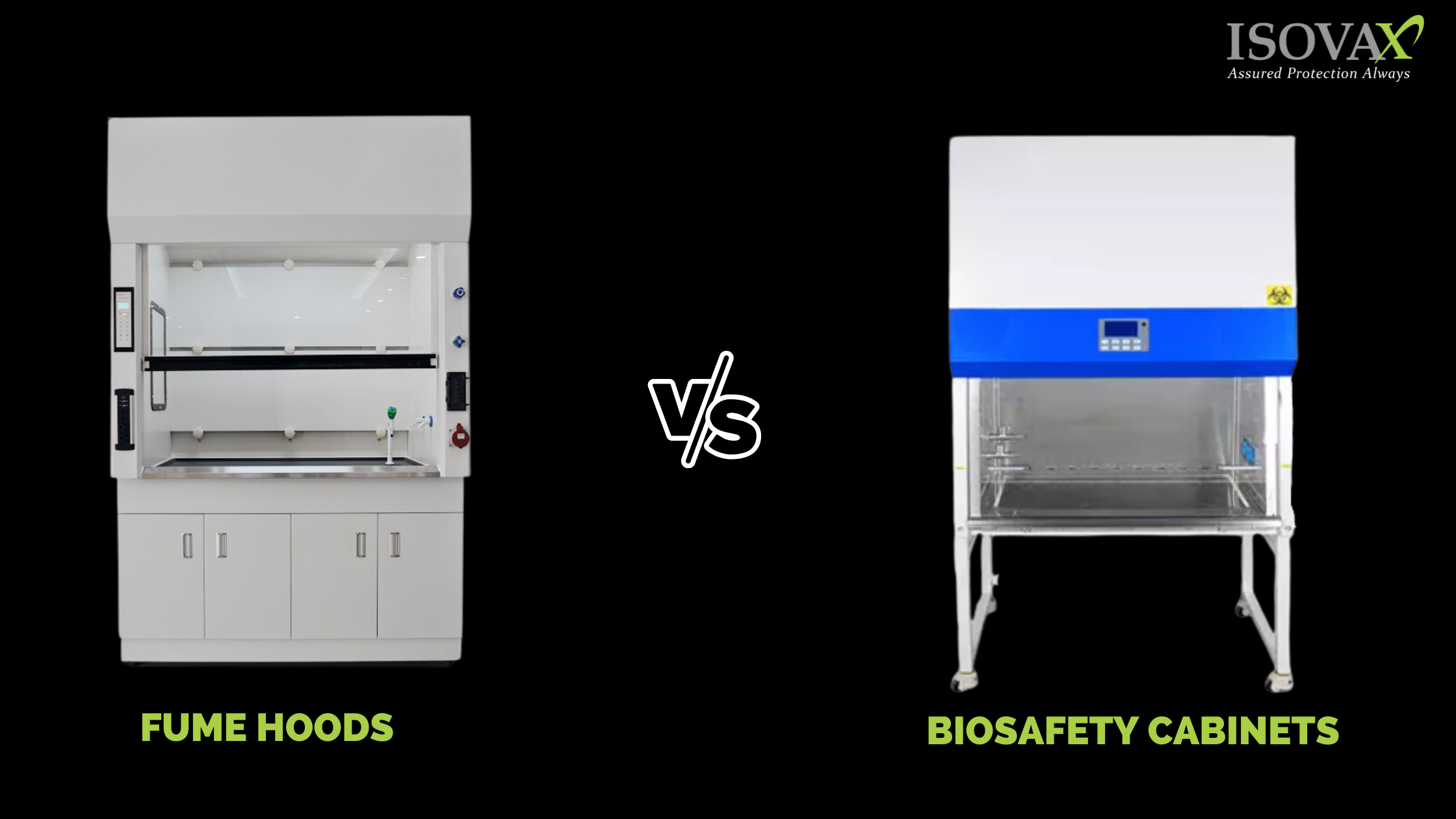
Fume hoods and biosafety cabinets are two examples of lab equipment that may purify the air in the work area and rid it of toxic compounds, allowing lab staff to handle hazardous materials without being concerned about contamination. They are both we...

Continuous development and integration of research findings and practical experiences are crucial for establishing standardized descriptions of cleanroom technology processes and specifications. The ISO standard family 14644 and VDI 2083 series of gu...
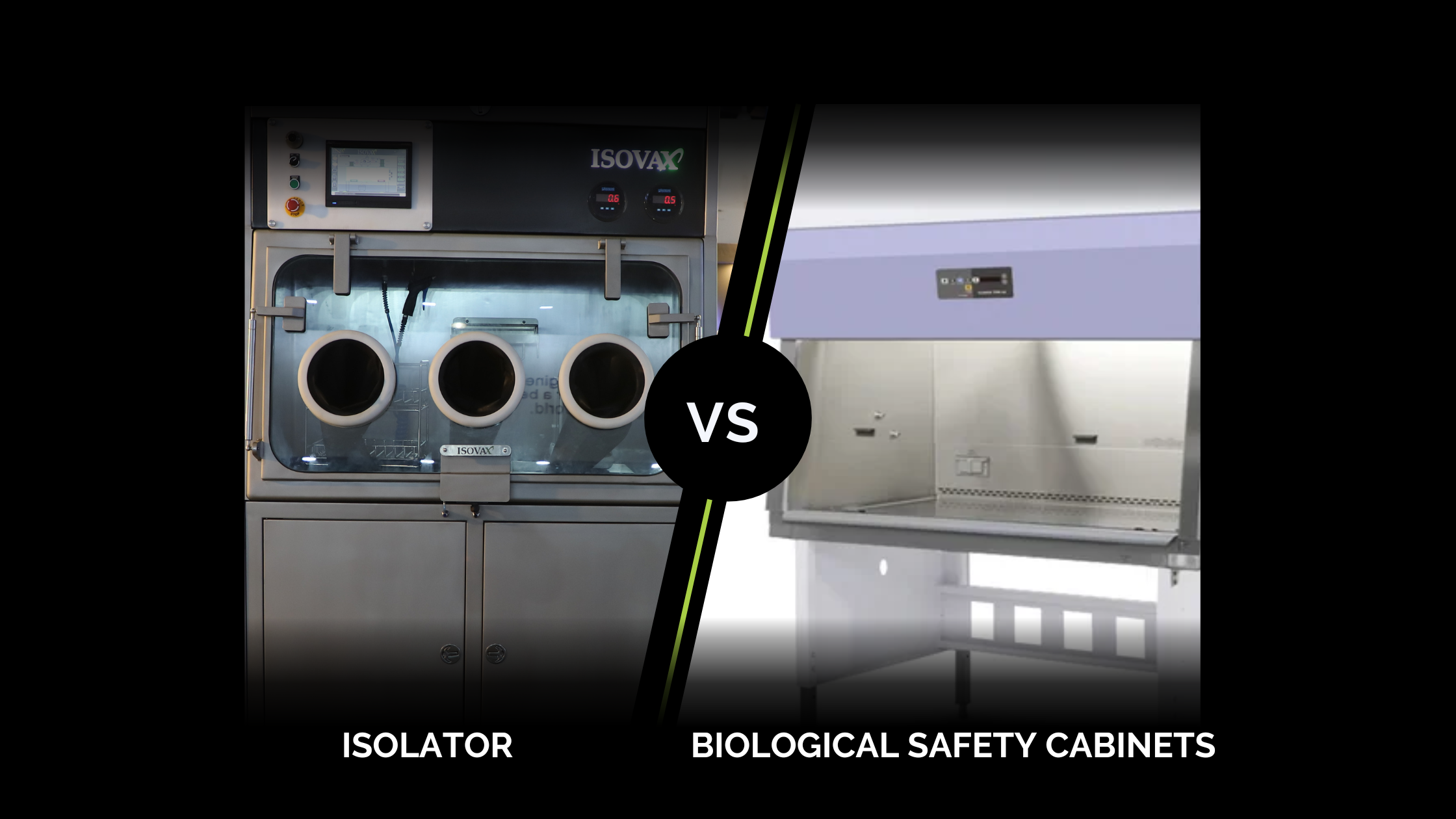
Biological Safety Cabinets (BSCs) and Isolators are two examples of containment cabinets frequently used in the pharmaceutical sector. These two tools were developed to protect lab personnel from breathing in hazardous substances and to stop contagio...

A laminar flow system is an essential concept in particulate contamination control. Laminar airflow is defined as air moving in the same direction at the same speed with no or minimal crossover of air streams (or “lamina”). Laminar flow i...

Isolators are important equipment in the pharmaceutical industry since they offer a protective barrier and confinement under aseptic settings. They’re made up of a series of physical barriers that employ confinement to provide a confined workin...

Every pharmaceutical manufacturer will eventually have to deal with the never-ending debate over RABS vs. isolator technology. It is critical to study the various options available to ensure that the barrier system fulfils the needs of the specific p...

In our previous articles, we spoke about the difference between RABS and Isolators and OEB and OEL Levels. Since we have got the basic understanding of how RABS are different from Isolators, we’ll compare and contrast two of the most prevalent ...
Understanding Sterile: Sterility in the context of pharmaceuticals refers to the absence of all viable microorganisms, including bacteria, viruses, and fungi. Achieving sterility is paramount to prevent contamination and ensure that medical products ...

Decontamination of high-containment laboratories is critical for controlling high-risk pathogens. All places with the potential to be contaminated must be disinfected in the case of an unintentional spill and as per the SOPs. While decontaminating bi...

Glove boxes, also known as isolation chambers or controlled atmosphere enclosures, are essential tools in various industries, including battery research and development. They provide a controlled environment with low levels of moisture and oxygen, al...

The leak rate is defined as the amount of air lost from a positive isolator or gained by a negative isolator per unit time. It is expressed as % volume loss per hour. Apart from % volume loss per hour, reciprocal hours, specific pressure decay......

How Precision Milling, Sifting, and Blending Isolators Improve Drug Safety in Pharmaceutical Production ? In drug production, being extraordinarily precise and safe is not an option. Milling, sieving, and blending are high-risk steps where product in...
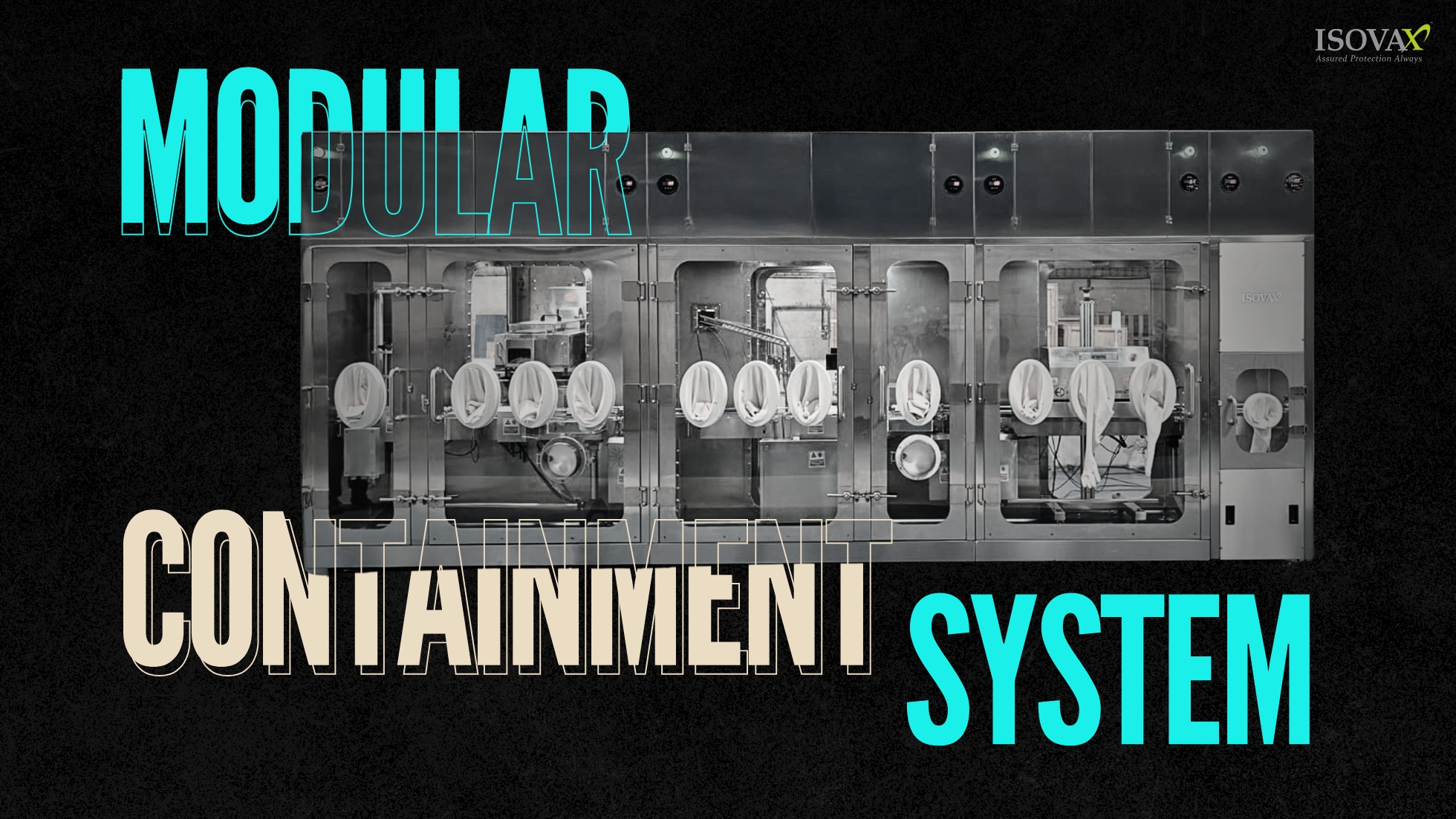
Why Modular Containment Systems Are the Future of Small-Batch and Pilot Manufacturing ? In the current pharmaceutical/biotech space, agility is critical. When running clinical trials, growing specialty (niche) therapies, or producing small-batch onco...

What Is a Nutsche Filter Isolator How is it used for HPAPI & Chemical Processing? In any pharmaceutical manufacturing operation, quality control and the safety of workers are critical factors. Working with high-potency active pharmaceutical ingre...

How Single-Use Flexible Isolators Improve Pharma Manufacturing In the rapidly evolving pharmaceutical manufacturing world, speed and sterility can no longer be treated as compromises. You need both without compromise. The necessity to protect the pro...

The appropriate selection and operation of contained dust collecting equipment is crucial in pharmaceutical facilities for a variety of reasons, ranging from environmental compliance and employee health & safety to production cleanliness & ef...

In recent years, isolation technology has advanced considerably. The technique is being used in the pharmaceutical business to improve the sterility assurance levels of aseptic products, but it is also finding a place in the medical device industry. ...

Many pharmaceutical drugs are light-sensitive and may change their properties when exposed to normal light directly. Some impurities could develop during this alteration, and the product might fail a quality control check. A sodium vapor lamp is a ga...

At extremely low airborne concentrations, Active Pharmaceutical Ingredients (API) have the potential to cause severe to significant health impacts in personnel. Containment devices or equipment should be used to control personnel exposure as part of ...

When it comes to containment for different pharmaceutical applications, such as API, formulation, or standalone use, Isovax is the brand to choose. We have discussed the VTD isolator that we recently made for a client in this blog. The wet mass that ...

In health care facilities, a novel low-temperature sterilization technology employs Vaporized Hydrogen Peroxide (VHP) to sanitize reusable metal and nonmetal instruments. Hydrogen peroxide vapor is used as a broad spectrum antimicrobial in the VHP st...

Personnel in pharmaceutical/ chemical manufacturing firms, particularly those who are exposed to a variety of chemicals, require safety while handling hazardous substance...

Powders and granulated solids are commonly used in the pharmaceutical industry. Handling these materials produces airborne dust, which can harm worker health and safety, be a nuisance, and/or result in product loss. This is especially true if the dus...
Why Modular Containment Systems Are the Future of Small-Batch and Pilot Man
How Single-Use Flexible Isolators Improve Pharma Manufacturing In the rapid
Benefits of Automation in Pharmaceutical Containment Systems Containment so
What Is a Nutsche Filter Isolator How is it used for HPAPI & Chemical P
How Precision Milling, Sifting, and Blending Isolators Improve Drug Safety
When it comes to containment for different pharmaceutical applications, suc
Understanding Sterile: Sterility in the context of pharmaceuticals refers t
Glove boxes, also known as isolation chambers or controlled atmosphere encl
In the realm of pharmaceuticals, precision and safety are paramount. One cr
Continuous development and integration of research findings and practical e
Fume hoods and biosafety cabinets are two examples of lab equipment that ma
VHP decontamination systems are becoming increasingly important as regulato
Restricted Access Barrier System (RABS) is a rigid barrier consisting of tr
In recent years, isolation technology has advanced considerably. The techni
Biological Safety Cabinets (BSCs) and Isolators are two examples of contain
It ought to be obvious saying that in order to reduce the danger of infecti
APIs are the compounds utilized in the production of pharmacological medica
A laminar flow system is an essential concept in particulate contamination
Powders and granulated solids are commonly used in the pharmaceutical indus
A Containment Strategy is a method for controlling workplace hazards
Relative humidity and absolute humidity are two different concepts, althoug
Many pharmaceutical drugs are light-sensitive and may change their properti
The appropriate selection and operation of contained dust collecting equipm
Decontamination of high-containment laboratories is critical for controllin
In health care facilities, a novel low-temperature sterilization technology
Isolators are important equipment in the pharmaceutical industry since they
In our previous articles, we spoke about the difference between RABS and Is
Personnel in pharmaceutical/ chemical manufacturing firms, p
Every pharmaceutical manufacturer will eventually have to deal with the nev
At extremely low airborne concentrations, Active Pharmaceutical Ingredients
The leak rate is defined as the amount of air lost from a positive isolator

