
29 Jul This is how Sodium Vapor Lamp saves photosensitive drugs!
Many pharmaceutical drugs are light-sensitive and may change their properties when exposed to normal light directly. Some impurities could develop during this alteration, and the product might fail a quality control check.
A sodium vapor lamp is a gas-discharge lamp that operates under an exciting condition to produce light with a characteristic wavelength of 589 nm. The main method by which a sodium vapor lamp functions is by using an electric arc to evaporate sodium metal. Other gases and substances will aid in turning on the bulb and regulating its color. These lamps are mostly employed for industrial and street lighting purposes.
The glass lamp is specifically made to firmly resist the hot sodium vapor action because sodium vapor has a very vigorous chemical reaction. In this case, maintaining the discharge tube’s precise temperature at around 300 degrees centigrade will maximize efficiency and minimize heat loss from the tube.
Working of Sodium Vapor Lamp:
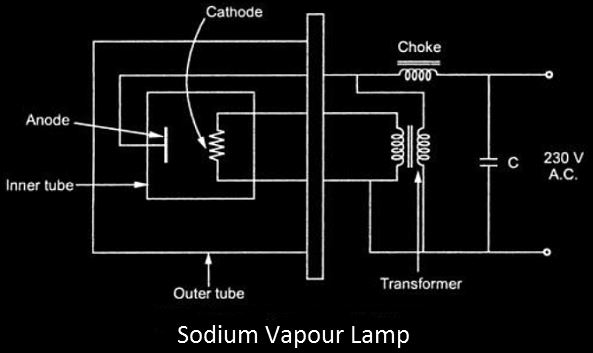
- When the sodium vapor lamp is turned on, it causes a discharge in the neon gas, which generates enough heat to cause the Na (Sodium) to evaporate.
- The cathode filament in the discharge tube is heated using a tiny transformer, while the anode terminal is linked directly to the 230V AC supply using a choke.
- The power factor (P.F) of the circuit described above will become extremely low due to the transformer and choke, so a capacitor is added to the circuit for rectification.
- Na (sodium) is deposited as solid sodium on the sidewalls of the tube when the vapor lamp is not in use.
- Na (sodium) is deposited as solid sodium on the sidewalls of the tube when the vapor lamp is not in use. Neon gas is expelled and produces red-orange hue light as soon as the vapor lamp is connected to the power source. Neon gas emissions generate heat, which transforms into solid sodium vapor. It begins producing all-yellow light after fifteen minutes.
- Neon gas emissions generate heat, which transforms into solid sodium vapor.
- It begins producing all-yellow light after fifteen minutes.
Application of Sodium Vapor Lamp in Pharma Industries:

In Pharma, energy of light can cause the activation of a drug’s molecules. The most common cause of photodegradation is the absorption of light with a short wavelength, between 500 and 300 nm. Lights include the chemical interactions that result in the formation of the new substance called “Impurity.”
Before creating the formulation, it is important to research how certain medications are impacted by light of particular wavelengths in order to shield the product from light. Pharmaceutical goods and APIs that are light-sensitive should be shielded from light deterioration during manufacturing. Excipients used in the formulation can lessen a light-sensitive drug’s sensitivity to light.
The monochromatic light produced by sodium vapor lamps is essential for spectroscopy and other types of chemical investigation in laboratories. Sodium Vapor Lamps can be used to set a reference point in spectrometers for spectroscopic applications. Low wavelength Sodium Vapor Lamps are ideal for evaluating light-sensitive medications in the pharmaceutical sector.
To know more about application of Sodium Vapor lamp in Pharma Industries, connect with us at sales@isovax.in



Sorry, the comment form is closed at this time.