
22 Dec The Ultimate Guide to Sterilization process in an Isolator.
In recent years, isolation technology has advanced considerably. The technique is being used in the pharmaceutical business to improve the sterility assurance levels of aseptic products, but it is also finding a place in the medical device industry. Isolators can be specially designed and manufactured to isolate a specific process, allowing for aseptic product manipulation without human interaction. Isolators satisfy predefined performance parameters, such as sterility assurance and have a minimum value range (SAL) < 10^(-6).
Vapor phase hydrogen peroxide is one approach for sterilizing an isolator.
About Hydrogen peroxide sterilization:
Hydrogen peroxide sterilization, also known as hydrogen peroxide gas sterilization, is a method of sterilizing heat-sensitive equipment/ products at low temperatures. A hydrogen peroxide sterilization cycle often takes less time than other sterilization methods, such as ethylene oxide sterilization. H2O2 vapor fills the sterilizer chamber, touching and sterilizing exposed device surfaces, in a hydrogen peroxide sterilization procedure.
Process of Sterilization in an Isolator:
The sterilization procedure is divided into four stages:
- The procedure starts with dehumidifying the air in the isolator chamber.
- In the second step, conditioning, hydrogen peroxide (H2O2) from a generator is vaporized and delivered at a high flow rate into the isolator. The isolator is typically at atmospheric pressure, although it can also be operated in a vacuum.
- The effectiveness of sterilization, which is the following step in the procedure, depends on the H2O2’s vapor dispersion efficiency and uniformity.
- An aeration procedure eliminates all H2O2 remnants from the isolator once sterilizing is finished.
The Cycle Development:
The maximum permissible H2O2 concentrations for isolators of various sizes are determined by taking temperature, relative humidity, load mass, and flow rate into account. Operators can use the tables to pick the appropriate cycle settings for the dehumidification, conditioning, and sterilizing phases.
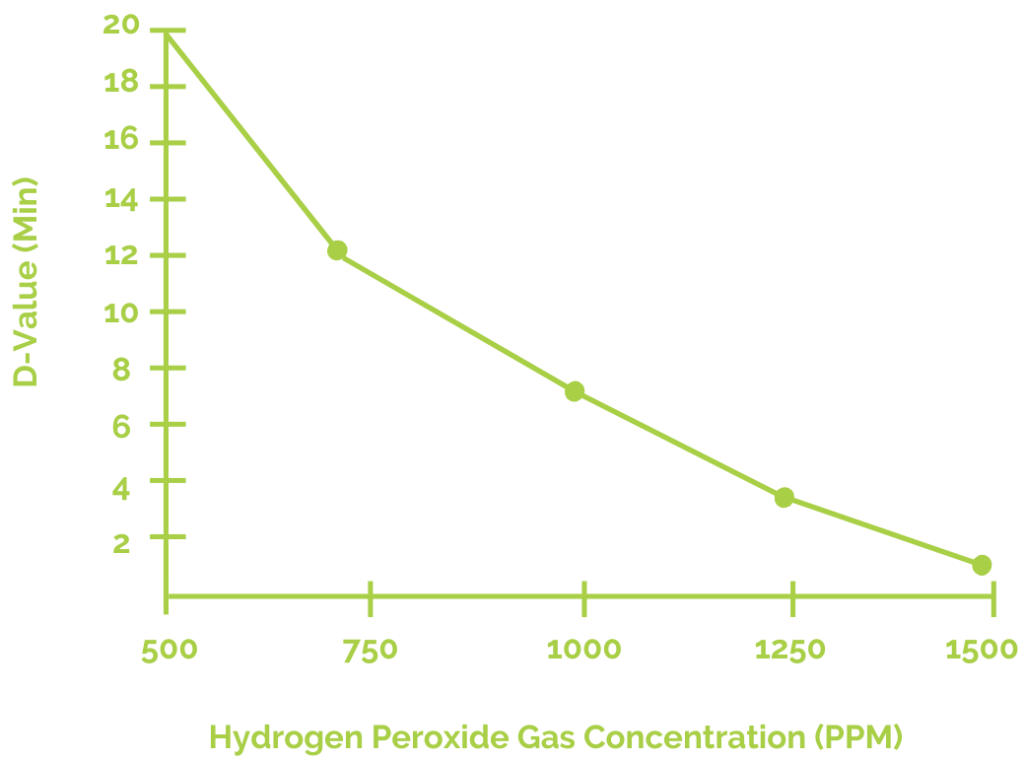
The D-value in sterilization refers to the time in minutes required to reduce a microbial population by 90%. Isolator operators can obtain approximate D-values for various cycle parameters to aid in the selection of optimal injection rates and H2O2 exposure durations. Most isolators have a D-value between 2 and 5 minutes.
After determining the right cycle settings, initial fractional H2O2 sterilization cycles can be undertaken utilizing Biological Indicators (BIs). The sterilization of all interior surfaces of the isolator, as well as the external surfaces of the items placed inside, is validated using resistant BIs, just like any other sterilization procedure. Bacillus stearothermophilus has been discovered as the bacterium with the highest resistance to H2O2 vapor sterilization. BIs with a spore population of 106 are utilized to validate to a SAL of < 10^(-6).
Operators select the best half cycle for H2O2 sterilization. This can be accomplished in one of two ways:
- Users can run sequential fractional cycles, increasing the gas-exposure period with each cycle, as is customary in EtO validation.
- During a single gas exposure cycle, users can test exposed BIs in duplicate at regular intervals.
Sterilization Validation:
After determining the half-cycle gas-exposure time, three successive cycles should be done using BIs and thermocouples. To effectively confirm the set cycle parameters, sterility testing should produce all negatives (no surviving BIs). All of the necessary BI growth controls should be negative, while all of the medium growth promotion tests should be positive.
Summing up:
The long-term preservation of isolator sterility should be maintained and constantly monitored. A routine sample regimen should be used for monitoring. Performing situation tests, such as losing power to the isolator or transferring extra supplies, should also be done during normal sample tests. Finally, because the addition of supplies is the principal route of contamination within the isolator, particular efforts must constantly be taken to guarantee that only sterile materials are added to the isolator.



Sorry, the comment form is closed at this time.