09 Oct Modular Containment Systems
Why Modular Containment Systems Are the Future of Small-Batch and Pilot Manufacturing ?
In the current pharmaceutical/biotech space, agility is critical. When running clinical trials, growing specialty (niche) therapies, or producing small-batch oncology drugs, agility and speed can mean the difference between success and a delay. This is where modular containment systems come into play, providing an innovative alternative for small-batch and pilot-scale production without sacrificing containment, compliance, or cost-efficiency.
Why Small-Batch Needs a Different Containment Philosophy
It is crucial to re-examine existing design and procurement processes as traditional large format containment solutions have never been designed for agility. These systems work tremendously well for high volume production, but when utilized for short run manufacturing, these systems inevitably create a bottleneck.
Small-batch and pilot production face unique challenges:
- Multiple product changeovers
- Short turnaround times
- Complex and potent APIs
- Evolving regulatory scrutiny even for trial batches
Modular containment systems are built to address these exact needs with the adaptability, speed, and cost-control that modern pharma demands.
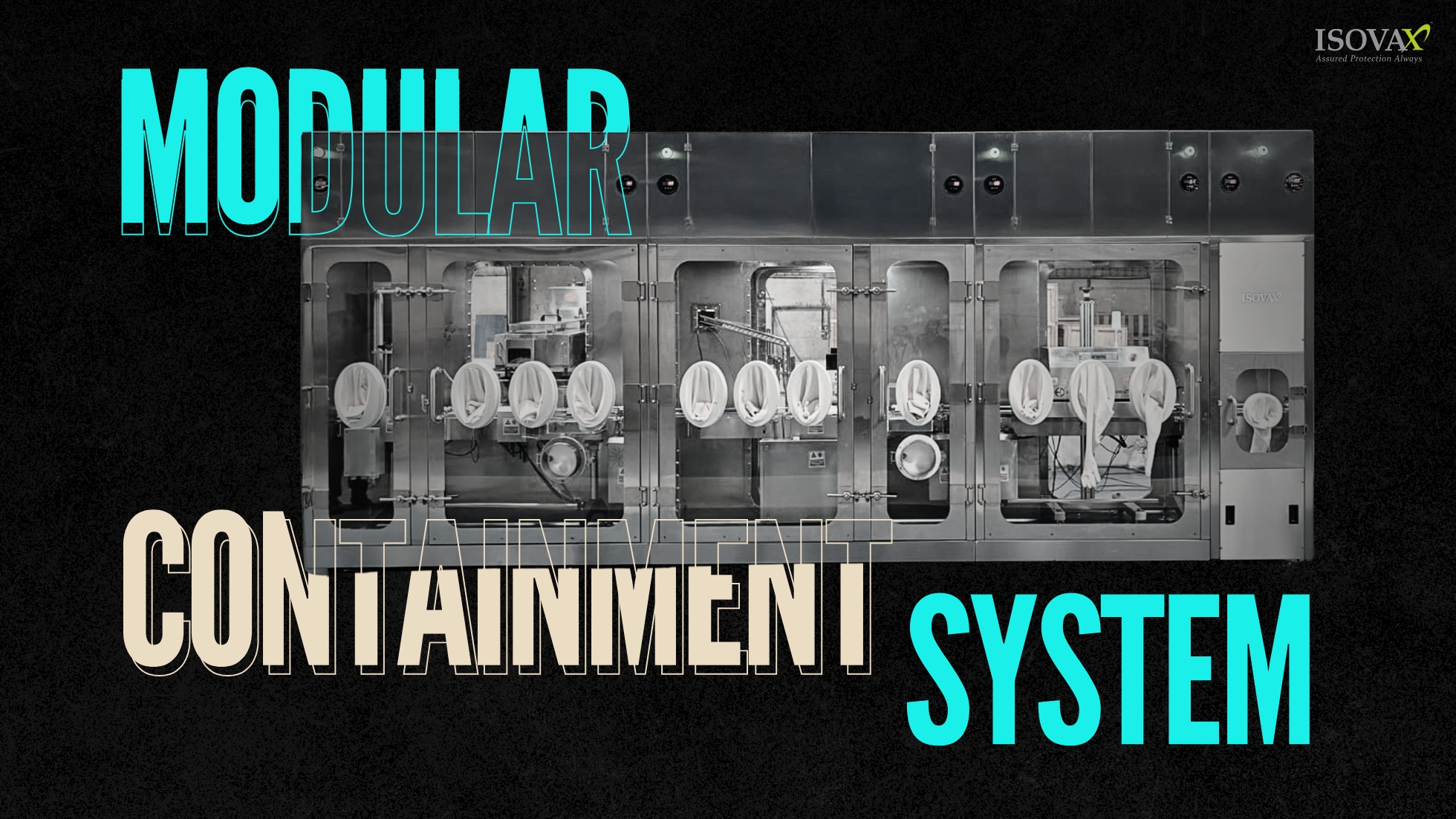
What Makes Modular Containment Systems Stand Out
Modular isolators are more than just smaller models of traditional isolators. They are designed with a plug-and-produce approach, intended for small space, changing product lines, and adaptable for rapidly growing biotech pipelines.
Scalable Configurations: From sterile filling to compounding to R&D work modular isolators can scale so you can add or subtract sections for your project.
Faster Setup, Quicker Validation: the time to design, fabricate and install is significantly less than that of rigid custom-built systems.
Reduced Downtime Between Batches: By integrating smart cleaning methods and single-use technologies, changeovers can be faster and safer.
Compact Footprint: Perfect for constrained facilities while staying cheaper in metropolitan, high-cost areas or temporary pilot facilities.
Built for Speed, Designed for Compliance
Let’s not forget about the elephant in the room, regulatory expectations. Because whether your working with HPAPIs or sterile compounds, compliance is not optional.
Modular containment systems are designed from day one with GMP, ISO and OEL expectations in mind. Many come pre-engineered with:
- HEPA filtration integrated
- Pressure cascade controls
- Rapid decontamination systems
- Glove ports & airlocks built-in for safe transfers
At that point you have not sacrificed flexibility for compliance, you get both!
Applications That Keep Evolving with You
What is most exciting about modular systems is their growth along with your process. A small batch isolator used today for cytotoxic R&D could later be utilized for scale-up or even commercial production through its reconfigurability.
Use Cases span:
- Oncology and hormone-based formulations
- Sterile injectables in early-phase trials
- Vaccine or biosimilar R&D
- Compounding hazardous drugs in the hospital setting
The Bottom Line: Faster Market Access and Smarter Investment
In small batch production, time is money and modular containment technologies save you on both. You don’t have to wait a year to build a custom facility. You don’t have to spend a fortune on equipment you will only use twice a year. You get speed, precision and compliance, all in a single flexible platform.
Is Modular Right for You? Let's Talk.
If you’re in the process of managing multiple products, planning a pilot, or are working with powerful APIs, then modular is probably the right way to go, and partnering with ISOVAX, you can design, deploy, and optimize your containment solution with care and in view of the future.
Get guidance on choosing the right modular system?
Talk to the Isovax experts. Our engineering team is ready to help pharma innovators like you make simplicity out of complexity module by module.



Sorry, the comment form is closed at this time.