
22 Aug Airflow in Compounding Aseptic Containment Isolators, Negative or Positive?
In the realm of pharmaceuticals, precision and safety are paramount. One critical process that underscores this principle is compounding aseptic containment. To achieve impeccable sterility and minimize the risk of contamination, the compounding aseptic containment isolator stands as a cornerstone technology. In this blog post, we delve into the world of compounding aseptic containment isolators, exploring their importance, features, and the role they play in ensuring the integrity of pharmaceutical products.
The Vital Role of Compounding Aseptic Containment Isolators
Aseptic compounding involves the meticulous preparation of sterile pharmaceutical products, such as intravenous medications, injections, and eye drops. Unlike many other sectors, even the tiniest of contaminants can have dire consequences in healthcare. This makes aseptic compounding not only a science but an art that requires precision, adherence to protocols, and the right tools.
Compounding aseptic containment isolators, commonly referred to as CACIs, are advanced pieces of equipment that facilitate the creation of sterile pharmaceutical products in a controlled environment. They provide a barrier between the operator and the product, preventing any external contaminants from entering and safeguarding the operator from any hazardous materials.
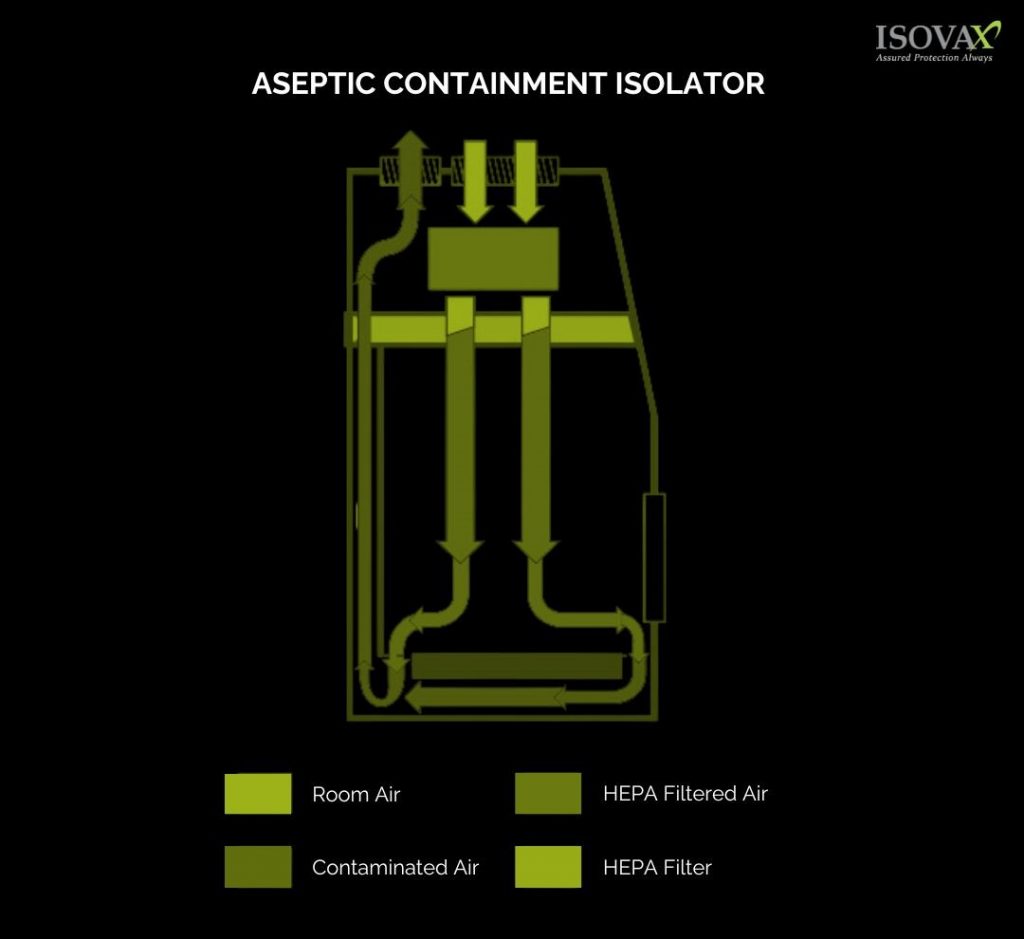
Key Features of Compounding Aseptic Containment Isolators
Sterile Environment:
CACIs create a sterile working area where the risk of contamination is minimized through the implementation of HEPA (High-Efficiency Particulate Air) filters and air circulation systems that remove airborne particles.
Physical Barriers:
These isolators are designed with glove ports, transfer ports, and viewing windows, allowing operators to interact with the substances being compounded without breaching the sterile environment.
Negative Pressure System:
CACIs often employ a negative pressure system, which ensures that any potential contaminants are drawn into the isolator and away from the operator, adding an extra layer of protection.
Airflow Management:
Advanced airflow systems maintain a continuous inward airflow, preventing airborne particles from entering the working area.
Glove Integrity Testing:
Some CACIs incorporate glove integrity testing to ensure that the gloves remain free from defects that could compromise the sterile environment.
Ensuring Operator Safety and Product Integrity
The primary focus of compounding aseptic containment isolators is to protect both the products being compounded and the operators themselves. Given that pharmaceutical products are often delicate and sensitive to external factors, maintaining their integrity is of utmost importance. CACIs drastically reduce the risk of contamination, which can lead to compromised product quality, recalls, and even harm to patients.
Moreover, the safety of the personnel operating these isolators is critical. Pharmaceutical compounds can range from simple vitamins to complex chemotherapy drugs, many of which can be hazardous if exposed to the operators. The physical barriers and air circulation systems of CACIs safeguard the operators from these potentially dangerous substances.
In a nutshell
In the world of pharmaceuticals, precision and safety are non-negotiable. Compounding aseptic containment isolators play an indispensable role in achieving these twin objectives. By creating a sterile and controlled environment, these isolators ensure that pharmaceutical products are prepared without the risk of contamination. Moreover, they protect the operators from exposure to potentially harmful substances, upholding safety standards in the workplace.
As technology continues to advance, we can expect even more sophisticated features to be integrated into these isolators, further enhancing their efficiency and effectiveness. Ultimately, compounding aseptic containment isolators stand as a testament to human ingenuity, as they enable the creation of life-saving medications with the utmost precision and care.
To know more about Airflow in Comounding Aseptic Containment Isolators connect with us at Sales@hvax.in



Sorry, the comment form is closed at this time.