

ISOVAX conducts Validation tests for all solutions provided, in compliance with the appropriate standards and testing guidelines. All containment devices must be accompanied with the basic qualification package, which includes DQ, IQ, OQ, and PQ.
Here’s the list of all the validation tests covered by ISOVAX:
Isovax, through Surrogate testing, aims to providing significant performance data prior to installation, that assists you in determining whether the isolators are compliant to the appropriate standards and capable of sufficing your requirement.
Surrogate testing is the use of a substitutes or surrogate compound that replicate an Active Pharmaceutical Ingredient (API) in order to validate the efficiency of dust containment alternatives for hazardous materials handling.
The test conditions are intended to imitate workplace activities as precisely as possible without the cost or health risks associated with handling the actual API.
Developing containment solutions for various products and dosage forms is just as important to us as optimizing processing ideas for highly active compounds according to their Operator Exposure Limits (OELs). From idea through execution and qualification, ISOVAX holds expertise in solids handling and liquid pharmaceutical filling, as well as Formulations and API containment facilities. Our team has extensive experience in the planning and implementation of containment projects.
For an existing facility, Isovax can help you extend the life of valuable, otherwise obsolete equipment, reduce the overall project cost and timescale, and optimizing your containment processes according to the respective OEL levels for:
This guarantees that you comply with the most recent industry rules and that your people, products and business receive maximum protection.
At ISOVAX, we are confident in our ability to facilitate technology transfers across the Globe, for the following reasons.
ISOVAX Offers End-To-End Technology Transfer Solutions based on Various Collaboration, Licenses, and Supply Models. Each development setup is distinct, based on the project’s goals and a thorough examination of the available information. Following consultation with in-house experts and close collaboration with you, we provide tailor-made solutions for your specific expectations of the targeted transfer.
A good validation system greatly reduces the risk of regulatory non-compliance. ISOVAX is backed by an experienced team of specialists that provide cleanroom validation services all around the world leveraging calibrated advanced equipment. The reports created are in the right forms, giving the customer a clear picture of any non-compliances, if any, with worldwide acceptable standards.
Our Clean room Validation Tests include:
OEB (Occupational Exposure Band) is a mechanism used to precisely assign chemicals into “categories” or “bands” based on their adverse health outcomes and potency considerations. It also aligns chemicals in groupings based on OEL (Occupational Exposure Limit) in order to establish safe handling guidelines.

Duo melius commodo lobortis cu, in pri labore expetendis
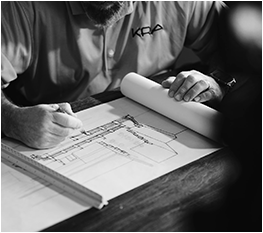
Eam ad euismod torquatos, ad vivendo commune eam


Eos ex iusto vitae iriure, nam iudico oporteat facilisi ad

Illum scriptorem reformidans pro cu, in putent urbanitas
Projects
Employees
Sites
Clients
Firms
Days

